神经内科Ken and Ruth Davey神经肿瘤科郑世远教授实验室的一项研究,发现了胶质瘤细胞中RNA剪接事件背后的新机制,这些机制或可作为新的治疗靶点。该研究结果发表在《临床研究杂志》上。
神经病学副教授、该研究的主要作者、医学博士肖松表示: “我们通过选择性剪接这一视角找到了一种治疗神经胶质瘤的不同方法,并发现了以前未被发现但对神经胶质瘤恶性肿瘤很重要的新靶点。”
胶质瘤是成人最常见的原发性脑肿瘤,起源于中枢神经系统并支持邻近神经元的神经胶质细胞。由于肿瘤的遗传和表观遗传异质性,胶质瘤对包括放疗和化疗在内的标准治疗具有高度耐药性,因此迫切需要寻找新的治疗靶点。
程实验室先前的研究发表在《癌症研究》杂志上,该研究表明,重要的剪接因子 SRSF3 在神经胶质瘤中与正常大脑相比显著升高,并且 SRSF3 调节的 RNA 剪接通过影响肿瘤细胞中的多种细胞过程来促进神经胶质瘤的生长和进展。
RNA剪接是一个涉及去除内含子(RNA的非编码区)和连接外显子(编码区)以形成支持细胞中基因表达的成熟mRNA分子的过程。
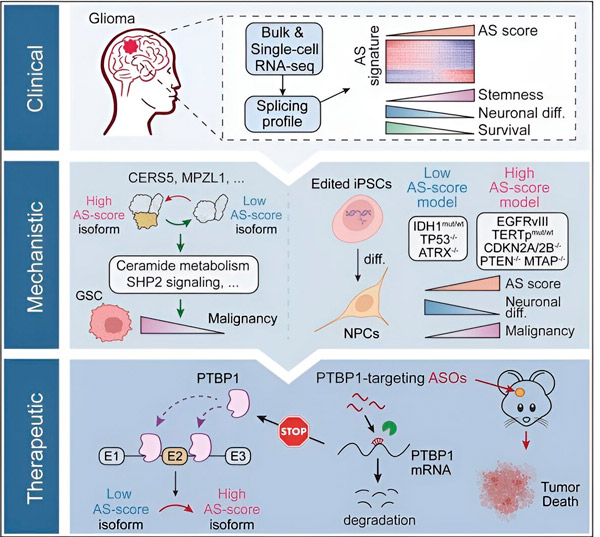
在目前的研究中,科学家旨在确定胶质瘤肿瘤细胞中可变剪接的改变、这些改变背后的机制,并确定它们作为治疗靶点的潜力。
研究人员利用计算方法和RNA测序技术,检测了患者样本中胶质瘤肿瘤细胞的剪接变化。为了证实这些变化,他们利用CRISPR基因编辑技术,将不同的胶质瘤驱动突变引入源自人类诱导多能干细胞(iPSC)的胶质瘤模型中。
他们发现,这些剪接变化因表皮生长因子受体 III (EGFRIII) 的变体而得到增强,已知该变体在包括神经胶质瘤在内的许多肿瘤中过度表达,而 IDH1 基因的突变会抑制该变体。
研究人员已经证实了两种 RNA 剪接事件的功能,这两种 RNA 剪接事件可以产生具有不同氨基酸序列的不同蛋白质异构体。
“这些亚型中只有一种能够促进肿瘤生长,而另一种亚型通常在正常大脑中表达。肿瘤利用这种机制选择性地表达促肿瘤亚型,而不是正常大脑亚型,”宋说。
研究小组随后分析了上游RNA结合蛋白,发现PTBP1基因调控胶质瘤细胞中促肿瘤RNA剪接。研究人员利用免疫缺陷小鼠的原位胶质瘤模型,采用基于反义寡核苷酸(ASO)的疗法靶向PTBP1,最终抑制了肿瘤的生长。
研究作者写道:“我们的数据强调了替代 RNA 剪接在影响神经胶质瘤恶性程度和异质性方面的作用,以及它作为成人神经胶质瘤治疗的弱点的潜力。”
宋说,研究人员的下一步是探索针对 PTBP1 引发抗肿瘤免疫反应的潜力。
“通过长读长RNA测序分析,我们发现靶向胶质瘤细胞中的PTBP1会导致产生多个在正常组织中不存在的剪接转录本。因此,我们的下一个项目是找出这种亚型是否能够产生一些抗原,以便免疫系统能够更好地识别肿瘤,”宋教授说。
宋还补充说,他们的团队对分析神经胶质瘤患者非肿瘤细胞(如免疫细胞)的剪接变化很感兴趣。
“我们已经知道剪接对于调节细胞功能非常重要,所以它不仅可以调节肿瘤的恶性程度,还可以调节免疫细胞的功能,以确定它们是否能够有效地杀死癌细胞。因此,我们也在对肿瘤浸润的免疫细胞进行一些生物信息学分析,看看免疫细胞浸润到肿瘤后,剪接是否发生了变化。
宋说:“我们的目标是确定可变剪接在塑造免疫抑制肿瘤微环境中的作用,并确定提高胶质瘤免疫疗法疗效的潜在目标。”

