德克萨斯大学西南医学中心的研究人员通过完全或部分消除 B 细胞中一种名为 midnolin 的蛋白质,成功抑制了具有遗传易患这些癌症的小鼠模型中的白血病和淋巴瘤。
他们的研究结果发表在《实验医学杂志》上,可能为这些疾病带来新的治疗方法,避免当前疗法的严重副作用。
“我们采用纯基因方法来寻找药物靶点,这个靶点非常引人注目,因为 B 细胞白血病和淋巴瘤高度依赖它,而大多数宿主组织却并不依赖它,”该研究的负责人、遗传宿主防御中心主任、德克萨斯大学西南医学中心免疫学和内科教授 Bruce Beutler 医学博士说。
贝特勒博士因发现免疫细胞中一组重要的病原体传感器(即 Toll 样受体)而荣获 2011 年诺贝尔生理学或医学奖。长期以来,他一直使用诱变法(通过接触一种名为 N-乙基-N-亚硝脲 (ENU) 的化学物质将突变引入动物模型的基因)作为研究基因功能的重要工具。
最近,Beutler 实验室开发了一种称为自动减数分裂作图 (AMM) 的方法,该方法可以通过追踪突变小鼠的异常特征来找到致病突变,从而识别维持正常生理状态所需的基因。
诱变常常会导致动物患上遗传疾病,通过研究动物体内的异常,可以深入了解受影响基因的功能。然而,正如Beutler博士所解释的那样,突变也可以提供对疾病的保护。
例如,一些突变可以保护艾滋病毒感染者或遗传性镰状细胞病患者免于出现症状。一些保护性突变背后的机制启发了治疗多种疾病的药物研发。
为了寻找针对免疫疾病的保护性突变,研究人员对突变小鼠进行了具有异常特征的免疫细胞筛选。在几组B细胞数量异常低的动物中(B细胞是适应性免疫系统中负责产生抗体的重要组成部分),研究人员利用AMM技术将缺陷追溯到midnolin(一种主要存在于B细胞中的蛋白质)的突变。
尽管完全缺乏 Midnolin 的动物会在出生前发育过程中死亡,但较温和的突变(包括一些通过基因技术引入的突变,允许该基因在成年期被删除)不会造成明显的伤害。
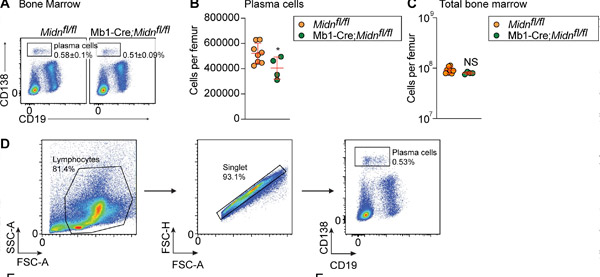
Mb1-Cre;Midn fl/fl 小鼠接种 TD 抗原 β-半乳糖苷酶后浆细胞的产生。(A 和 B) 8 周龄 Mb1-Cre;Midn fl/fl 和 Midn fl/fl 小鼠接种 β-半乳糖苷酶后骨髓中浆细胞的代表性流式细胞术图 (A) 和数量 (B)。(C) 每根股骨的总骨髓细胞数。(D) 浆细胞分离策略。来源:《实验医学杂志》(2024)。DOI:10.1084/jem.20232132
研究人员显著降低或消除了易患B细胞白血病和淋巴瘤(B细胞分裂失控导致的癌症)的小鼠体内的midnolin水平。尽管midnolin水平正常的小鼠在5个月内死于这些疾病,但大多数midnolin水平较低或完全没有的小鼠从未患上恶性肿瘤。
进一步的实验表明,midnolin在B细胞中的作用是刺激蛋白酶体的活性,蛋白酶体是细胞器,负责处理受损或不再需要的蛋白质。Beutler博士解释说,目前用于治疗B细胞白血病和淋巴瘤的一些疗法是通过抑制蛋白酶体活性来发挥作用的,就像去除midnolin一样。
然而,与这些具有许多潜在严重副作用的药物不同,在动物模型中消除或减少米诺林似乎不会产生任何负面影响。
未来的研究将集中于开发抑制米诺林的药物,这可能最终成为治疗 B 细胞癌症的新基础。

