
瑞典的研究人员表示,他们已经完善了将普通皮肤细胞转化为神经干细胞的技术,他们表示,这使他们更接近于开发出价格合理的针对阿尔茨海默病和帕金森病的个性化细胞疗法。
研究团队利用定制设计的微流体装置,开发出一种前所未有的加速方法,将人类皮肤细胞重新编程为诱导多能干细胞(iPSC),然后将其转化为神经干细胞。
该研究的第一作者Saumya Jain表示,该平台可以通过提高细胞的兼容性和被患者身体接受度来改善细胞治疗并降低成本。这项研究由瑞典皇家理工学院的科学家发表在《先进科学》杂志上。
该研究的资深作者安娜·赫兰德 (Anna Herland) 表示,这项研究首次展示了利用微流体技术引导 iPSC 成为神经干细胞。
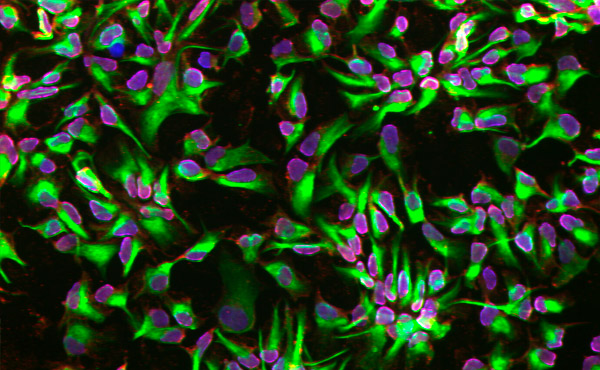
利用微流体平台分化的神经干细胞。图片来源:瑞典皇家理工学院
正常细胞转化为神经干细胞实际上是一个两步过程。首先,将细胞暴露于生化信号,诱导它们成为多能干细胞(iPSC),从而可以产生不同类型的细胞。
然后,它们被转移到一种模拟神经系统形成过程中信号和发育过程的培养物中。这一步骤被称为神经分化,它将细胞重新定向为神经干细胞。
在过去十年中,这类工作的实验室环境已逐渐从传统的平板电脑转向微流控设备。Herland 表示,新平台代表了 iPSC 生成和神经干细胞分化这两个步骤中微流控技术的进步。
研究人员利用来自人类皮肤活检的细胞发现,与传统培养皿中分化的细胞相比,微流体平台可以在更早的阶段加速细胞向神经命运的分化。
赫兰德说:“我们已经证明,微流体平台的密闭环境增强了产生神经干细胞的承诺。”
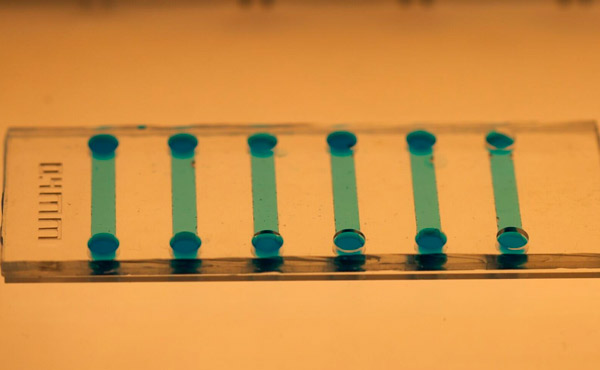
用于诱导干细胞的微流控芯片特写。图片来源:瑞典皇家理工学院
Jain 表示,微流体芯片很容易利用聚二甲基硅氧烷 (PDMS) 制造,而且其微观尺寸可以显著节省试剂和细胞材料。
他补充道,该平台可以轻松修改,以适应分化成其他细胞类型。它可以实现自动化,提供一个封闭的系统,确保高度均质细胞群生产的一致性和可靠性。
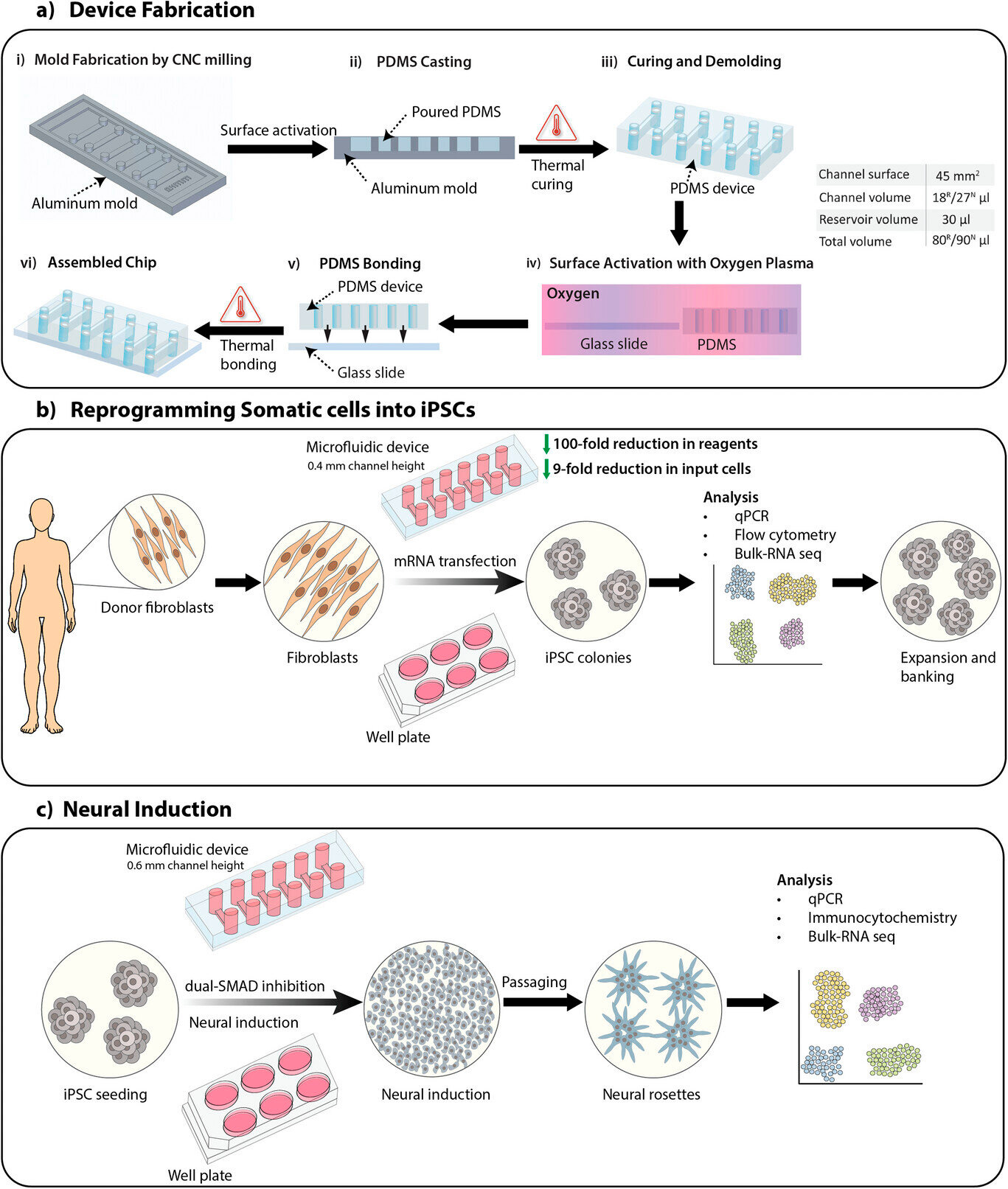
研究概述,包括器件制造、体细胞重编程为诱导多能干细胞 (iPSC),以及使用 SMAD 双重抑制方案诱导 iPSC 分化为神经干细胞。A
) 分别用于体细胞重编程 (R) 和神经诱导 (N) 的微流控器件制造过程,通道高分别为 0.4 毫米和 0.6 毫米。通道体积和总体积列于表中。B
) 使用 mRNA 转染在微流控器件和微板上将体细胞重编程为 iPSC 的过程概述。C
) 使用 SMAD 双重抑制方案在微流控器件和微板上将 iPSC 诱导为神经干细胞的过程概述。
来源:Advanced Science (2024)。DOI:10.1002/advs.202401859
Jain 补充道:“这是朝着实现针对阿尔茨海默病和帕金森病的个性化细胞疗法迈出的一步。”
这项研究还涉及卡罗琳斯卡医学院和隆德大学的科学家,他们与 IndiCell 联盟合作。

