Scientists modified E. Coli with parts of the HIV virus to develop a successful vaccine
最近審查:14.06.2024
Nikolay Shcherbak, assistant professor of biology at Örebro University, has just returned to Sweden after attending a conference in South Africa, where he presented research that increases the chances of developing an HIV vaccine. Together with other researchers, he genetically modified the probiotic bacterium E. Coli by adding part of the HIV virus to it.
The article was published in the magazine Microbial Cell Factories.
“Using advanced technology, we insert DNA sequences into specific locations in bacteria. We use a part of the HIV virus that is not infectious, but still causes the body to produce neutralizing antibodies,” says Shcherbak.
E. Coli bacteria live in the intestines of humans and other animals, and some variants cause different types of infections. However, there are also beneficial variants of these bacteria that can help improve your gut microflora. One of these bacteria, probiotic E. Coli strain Nissle, was used by researchers from Örebro in their study.
“The bacteria we use are sold as dietary supplements in Germany, but as far as I know they are not available in Sweden. These supplements are recommended for people with irritable bowel syndrome (IBS) or other stomach disorders."
HIV is a virus that can lead to the fatal immunodeficiency disease AIDS, for which there is no cure. However, there are drugs to treat HIV that allow infected people to live without symptoms or risk of transmitting the disease.
“An HIV-infected person must take antiretroviral drugs for the rest of his life, and their cost may be unaffordable for everyone. Researchers have been developing a vaccine for many years, but, unfortunately, this is not a priority for pharmaceutical companies,” says Shcherbak.
If the bacteria developed at Örebro University lead to an approved pharmaceutical product, it could be taken in tablet form. Vaccines in tablet form have significant advantages over vaccines that must be injected. The tablets are simpler and more convenient to use and do not need to be stored at low temperatures like some COVID-19 vaccines.
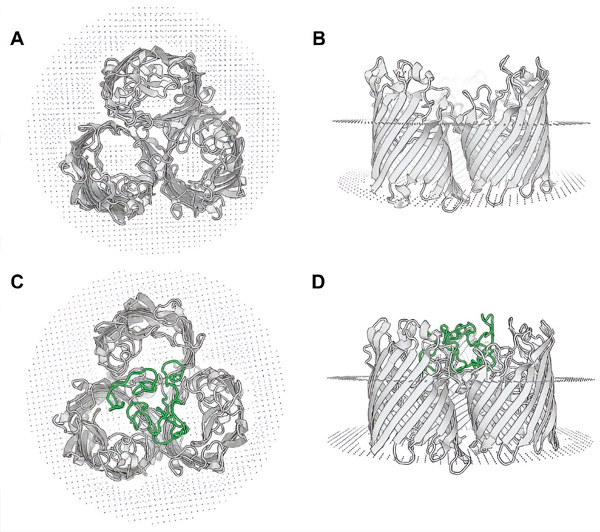
Homologous modeling of the recombinant protein OmpF-MPER. Top (A) and side (B) views of the OmpF protein trimer from E. Coli strain K-12 (based on 6wtz.pdb). Top (C) and side (D) views of the predicted protein OmpF-MPER from EcN-MPER, homology modeling performed on the structure of 6wtz.pdb using the SWISS-MODEL tool. The location of the MPER sequence is indicated in green. Source: Microbial Cell Factories (2024). DOI: 10.1186/s12934-024-02347-8
In many previous attempts to use bacteria to make vaccines, researchers have used antibiotic resistance genes to preserve genetic modifications in bacteria. However, this method can lead to negative consequences such as antibiotic resistance, which is a growing global public health problem. Using CRISPR/Cas9 technology, researchers from Örebro have created a stable genetic modification in probiotic bacteria without the need for antibiotic resistance genes.
Shcherbak does not see any risks in using genetically modified bacteria. However, more research is needed, including animal testing, before the technology is tested in humans and the vaccine can see the light of day.
“It takes at least a couple of years to prepare and obtain ethical approvals. Under normal conditions, drug development takes about ten years,” says Shcherbak.

