By studying modifications of the immune system of tumors throughout the day, scientists from the University of Geneva and the Ludwig Maximilian University of Munich demonstrate their impact on the diagnosis and treatment of patients.
The most promising antitumor treatments currently are immunotherapy, which are aimed at strengthening the action of the patient's immune system in the fight against cancer. However, although these methods are highly effective in some cases, their success is sometimes disappointing. How can this variability be explained?
In previous studies, a team from the University of Geneva (UNIGE) and the Ludwig Maximilian University of Munich (LMU) discovered the importance of the rhythmicity of the immune system for tumor growth. These same scientists have now shown that the immune profile of tumors varies significantly depending on the time of day when the biopsies are performed.
These temporary changes can lead to misdiagnosis and inadequate treatment. In addition, some previously overlooked therapeutic targets may prove key to combating the disease. These findings, published in the journal Cell, could have significant implications for clinical care and drug research.
In 2022, a research team led by Christoph Scheuermann, professor at the Department of Pathology and Immunology and the Center for Inflammation Research at the UNIGE Faculty of Medicine and the University of Munich, observed an unexpected phenomenon: the growth and severity of tumors are linked to the circadian rhythm of immune cells. "But to use these results in a clinical context, we needed to understand their details in a model that was close to reality," says Scheierman.
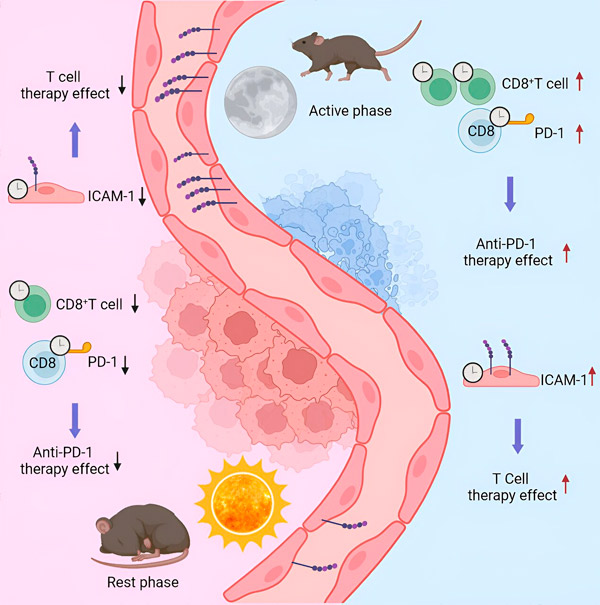
To do this, scientists injected a group of mice with melanoma cells, and then collected the resulting tumors at different times of the day after two weeks. Depending on the time of day and therefore the immune activation of the animal, the number of immune cells, as well as their type and characteristics, varied significantly. This may have important implications in clinical settings.
Source: Cell (2024). DOI: 10.1016/j.cell.2024.04.015
"In the hospital, patients undergo a biopsy to identify the tumor and its immune characteristics," explains Scheierman. "Treatment, and in particular immunotherapy, is then determined based on this examination. Now, depending on the timing of the biopsy, the number of infiltrated immune cells can be very high - and the tumor is classified as 'hot' - or very low ('cold'), although they are the same tumor, performing a biopsy at the wrong time can lead to a misdiagnosis.
A look at the timing of immunotherapies
To get as close to clinical reality as possible, the scientists applied two approved and widely used treatments to their groups of mice: CAR-T cells (designed specifically to recognize and target proteins specific to tumor cells) and immune checkpoint inhibitors. Which suppress the immune system's natural brakes to increase its activation against tumors.
"When applied at the wrong time, these treatments had no effect. At the right time, the tumor burden could be significantly reduced," explains Scheierman. "The number of immune cells present or absent in the tumor is a factor, but their characteristics and behavior are also important."
Indeed, depending on the modulation of the molecular elements used to create these treatments, the timing of their application becomes key. At the right time, the cells to be destroyed are immediately recognized. At the wrong time, the target molecules have lower expression and the drug has no effect.
Adaptation of treatment schedules and methods
These studies, conducted in mice, are supported by analysis of patient survival rates after immunotherapies. Morning treatment—at the peak of immune activation in humans—is systematically associated with better survival rates. Studies are planned to evaluate the impact of changes in screening and treatment timing on patients. Other projects will explore potential drug targets that have so far been underestimated.
In addition, these discoveries about immune rhythms have even broader implications: from the point of view of personalized medicine, on the one hand, to adapt therapeutic approaches to the temporal profiles of patients (in 10-20% of people the biological rhythm does not coincide with the general population), and in the context of other pathologies, especially autoimmune diseases.

