法兰克福歌德大学的一项研究指出,沙利度胺衍生物可能用于治疗癌症。沙利度胺在20世纪50年代曾作为安眠药出售。后来,它因在妊娠早期导致严重的出生缺陷而臭名昭著。
沙利度胺分子还能标记细胞内需要破坏的蛋白质。作为当前研究的一部分,科学家们合成了沙利度胺的衍生物。他们能够证明这些物质能够影响癌细胞存活所需的蛋白质的破坏。
或许没有任何其他分子像沙利度胺一样有着如此动荡的过去。它是20世纪50年代一种被许多国家批准用于镇静和安眠的药物的主要成分。但很快人们就发现,服用沙利度胺的孕妇经常会生下有严重畸形的婴儿。
然而,近几十年来,医学界再次对它寄予厚望。研究表明,它能抑制血管生长,因此可能适合用于切断肿瘤与营养介质的联系。此外,它在治疗多发性骨髓瘤(一种骨髓恶性肿瘤)方面也被证明非常有效。
“我们现在知道,沙利度胺可以被称为‘分子胶’,”法兰克福歌德大学药物化学研究所的程兴来博士解释说。“这意味着它能够抓住两种蛋白质并将它们连接在一起。”
这尤其有趣,因为其中一种蛋白质是一种“标签机”:它将明确的“垃圾”标签附加到另一种蛋白质上。
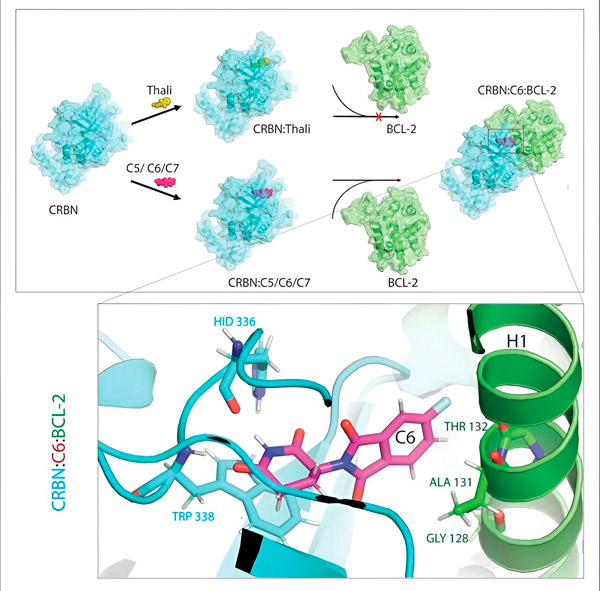
沙利度胺衍生物 C5、C6 和 C7 可以改变 CRBN(即“标记机器”),使其能够与 BCL-2 结合。这样,BCL-2 分子就被标记并降解——这可能是一种抗癌新策略。作者:程兴来博士。
细胞的废物处理系统能够识别这种标记:它会抓住标记的蛋白质分子并将其粉碎。“这种机制解释了沙利度胺的不同作用,”程教授说。“根据标记的蛋白质类型,它可以在胚胎发育过程中导致畸形,也可以破坏恶性细胞。”
这种机制为医学带来了巨大的潜力,因为癌细胞依赖某些蛋白质才能存活。如果能够系统地靶向并粉碎癌细胞,或许就能治愈癌症。问题在于,这种分子胶水非常特殊。
它的一个结合伙伴始终是细胞的标记机器,或者用科学术语来说,是一种名为 CRBN 的 E3 连接酶。在体内成千上万种蛋白质中,只有极少数可以成为第二个伙伴——具体是哪些取决于胶水。
“所以我们创造了一系列沙利度胺衍生物,”程教授说。“然后我们测试它们是否具有粘附特性,如果具有,它们对哪些蛋白质有效。” 为了做到这一点,研究人员将这些衍生物添加到培养细胞系中的所有蛋白质中。然后他们观察了哪些蛋白质在CRBN存在下被降解。
“在这个过程中,我们发现了三种衍生物,它们可以标记一种对降解至关重要的细胞蛋白——BCL-2,”Cheng解释说。“BCL-2会阻止细胞启动自我毁灭程序,所以如果没有它,细胞就会死亡。”
正因如此,BCL-2 长期以来一直是癌症研究的焦点。甚至有一种名为维奈克拉的白血病药物,可以降低 BCL-2 的效力,从而导致突变细胞自毁。
“然而,在许多癌细胞中,BCL-2 本身发生了突变。因此,维奈克拉不再抑制这种蛋白质,”程教授说道。“我们能够证明,我们的衍生物也能标记这种突变形式,以便降解。此外,我们在马克斯·普朗克生物物理研究所的合作伙伴在计算机上模拟了沙利度胺衍生物与 BCL-2 的相互作用。结果表明,这些衍生物的结合位点与维奈克拉完全不同——这一结果后来得到了我们实验的证实。”
研究人员还在携带癌细胞的果蝇身上测试了他们的化合物。经此治疗的果蝇的存活率显著提高。然而,程教授提醒大家不要抱有太大希望,因为这些结果仍处于基础研究阶段。“虽然它们表明改良的沙利度胺分子具有巨大的治疗潜力,但我们还不能断言它们是否会在某个时候在实践中得到证实。”
研究结果发表在《细胞报告物理科学》杂志上。

