新的研究让我们对大脑独特的左右差异背后的遗传机制有了更好的了解,为更好地理解与大脑异常不对称相关的人类疾病铺平了道路。
伦敦大学学院、惠康基金会桑格研究所、牛津大学及其他合著者的研究人员发现,一种名为Cachd1的蛋白质在大脑两侧不同神经结构和功能的形成中起着关键作用。这项研究发表在《科学》杂志上。
通过对斑马鱼进行基因实验,研究人员发现,当Cachd1发生突变时,大脑右侧会失去正常的不对称发育,变成左侧的镜像。这种疾病会导致神经连接异常,从而影响大脑功能。
这一发现揭示了大脑不对称的遗传机制。大脑不对称是许多动物物种(包括人类)中观察到的现象。了解这些过程可能有助于更好地理解人类大脑不对称紊乱导致的疾病,例如精神分裂症、阿尔茨海默病和自闭症谱系障碍。
尽管人类大脑的左右半球在解剖学上呈镜像关系,但它们的功能存在差异,这会影响神经连接和语言等认知过程。这些神经回路的左右差异是如何产生的,目前仍不清楚。
研究人员利用斑马鱼(因其透明胚胎而成为研究大脑发育的知名模型生物)开始研究 Cachd1 如何影响大脑不对称。
研究小组发现,当 Cachd1 发生突变时,大脑中一个名为缰核的区域会失去正常的左右区分。右侧的神经元变得与左侧的神经元相似,这会破坏缰核中的神经连接,并可能影响其功能。
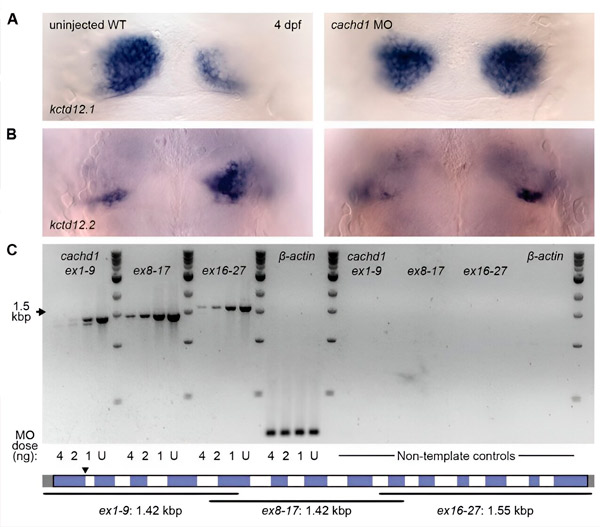
使用吗啉基寡核苷酸敲低 cachd1 基因导致双侧对称性。(AB) 未注射野生型幼虫和注射 cachd1 吗啉基寡核苷酸的幼虫受精后 4 天的背视图,使用反义核糖核酸探针对不对称背侧缰核标记 kctd12.1 进行整体原位杂交。(C) cachd1 转录本的半定量 RT-PCR。来源:Science (2024)。DOI:10.1126/science.ade6970
蛋白质结合实验表明,Cachd1 与两种受体结合,使细胞能够通过 Wnt 信号通路进行通讯,Wnt 信号通路是研究最深入的细胞通讯通路之一,在早期发育、干细胞形成和许多疾病中发挥重要作用。
此外,Cachd1 的作用似乎特异性地作用于大脑右侧,这表明存在一个未知的抑制因子限制其在左侧的活性。尽管全部细节尚不清楚,但数据强烈表明,Cachd1 通过特异性地调节右侧的细胞通讯,在确定发育中大脑左右两侧的区别方面发挥着关键作用。
未来的研究将探讨Cachd1是否具有与Wnt通路相关的其他重要功能。
“这是一个高度合作的项目,极大地受益于跨学科方法——遗传学、生物化学和结构生物学结合在一起,以更好地了解大脑左右不对称的建立,并确定在健康和疾病中发挥多种作用的重要信号通路的新组成部分,”该研究的共同作者、前威康桑格研究所博士生、现任伦敦大学学院细胞和发育生物学系成员的 Gareth Powell 博士说。
“我很高兴看到这项高度合作的研究成果发表,它汇集了来自不同机构、拥有不同研究兴趣和技能的众多才华横溢的人才。团队的共同努力使我们能够在Wnt信号通路和大脑不对称的形成方面取得令人兴奋的新发现,”该研究的资深作者、伦敦大学学院细胞与发育生物学系成员史蒂夫·威尔逊教授说道。

