New horizons in early cancer detection: multicancer tests (MCED) and their prospects
最近審查:14.06.2024
Cancer remains one of the most serious public health problems, causing significant mortality worldwide. In 2022 alone, there were approximately 19.3 million new cancer cases and 10 million cancer-related deaths worldwide. The high mortality rate is primarily associated with late detection of the disease, often after it has metastasized, when treatment options are limited. Early detection is key as it could prevent at least 15% of cancer deaths within five years by allowing precancerous lesions to be removed and localized forms of the disease treated.
Cancer is characterized by the uncontrolled proliferation and proliferation of abnormal cells in the body. While normal cells undergo a regulated process of growth and division, old or damaged cells naturally die and are replaced by new ones. However, when this process is disrupted, it can lead to the formation of tumors, which can be either benign or malignant. Malignant tumors, unlike benign tumors, invade nearby tissue and spread to other parts of the body through metastasis, which is responsible for the majority of cancer-related deaths.
Recent advances in cancer research have led to the development of multicancer early detection (MCED) tests. These tests represent a promising approach to detect cancer at its earliest stages by analyzing tumor-related markers in body fluids such as blood and using artificial intelligence to detect and differentiate between different types of cancer. MCED tests are part of a broader category of liquid biopsies that are non-invasive and cost-effective alternatives to traditional tissue biopsies. They provide a comprehensive genomic picture of the tumor, allowing the detection of specific biological signals in the DNA, RNA or proteins released by cancer cells.
Research on this topic was published in the Journal of Exploratory Research in Pharmacology.
MCED tests offer several advantages, including noninvasiveness, cost-effectiveness, and the ability to conduct serial sampling to monitor drug resistance and tumor progression. These tests detect fragments of DNA or RNA released by tumor cells into the bloodstream, helping to determine the most likely origin of the cancer. This capability is key to detecting cancer at an early stage, when it is most treatable.
Liquid biopsies, the basis of MCED tests, have revolutionized the approach to cancer detection. Traditional biopsies, which involve surgical removal of tissue, can be invasive, painful and come with risks of complications. In contrast, liquid biopsies only require a blood sample, making the process significantly less invasive and more acceptable to patients. This method not only improves patient comfort, but also allows for repeat sampling over time, providing continuous monitoring of cancer progression or response to treatment.
In addition, liquid biopsies can better capture tumor heterogeneity than a single tissue biopsy because they collect genetic information from cancer cells released into the bloodstream from multiple sites in the body.
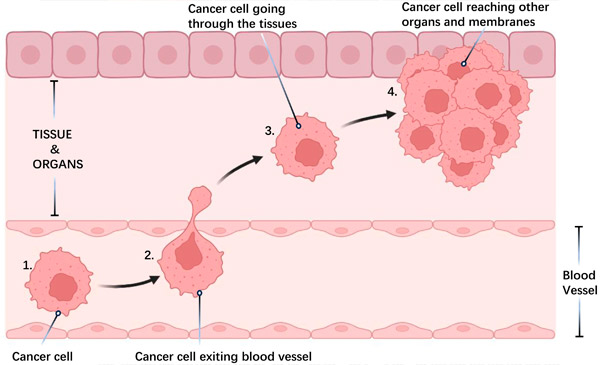
Cancer cell metastasis:
1) Cell detachment: Cancer cells leave the primary tumor and invade nearby tissue.
2) Vascular entry and travel: Cells enter blood or lymphatic vessels, spreading throughout the body.
3) Attachment to tissues: Cells attach to new tissues.
4) Formation of distant tumors: New tumors develop at distant sites.
Metastasis, the spread of cancer cells from the primary tumor to other organs, is the leading cause of cancer deaths. This process involves various cellular mechanisms, such as infiltration into adjacent tissues, evasion of detection and suppression of the immune system, influence on the local tissue environment, and development of treatment resistance.
Source: Journal of Exploratory Research in Pharmacology (2024). DOI: 10.14218/JERP.2023.00007
Despite their potential, MCED tests face significant challenges in clinical implementation, including the need for a standardized system to evaluate their effectiveness and safety. Currently, only a few MCED tests are available to clinicians, and none have been approved by the Food and Drug Administration (FDA) for market release. The specificity of these tests is usually high, but their sensitivity may vary depending on the type and stage of cancer.
The lack of standardized protocols for evaluating MCED tests is a barrier to their widespread implementation. Each test uses different methodologies, biomarkers, and analytical techniques, making it difficult to compare study results or establish universal performance metrics. To address this issue, regulatory agencies and research institutions must collaborate to develop comprehensive guidelines to ensure the reliability and accuracy of MCED tests. This standardization is critical to obtain regulatory approval and integrate these tests into routine clinical practice.
MCED tests can be used both for symptomatic patients to minimize delays in diagnosis, and for screening apparently healthy people for asymptomatic cancers. Liquid biopsies, which are the basis of MCED tests, have shown promise in clinical trials, providing a non-invasive means for detecting and monitoring cancer. The US Surveillance, Epidemiology, and End Results Program used state transition models to predict the potential benefits of MCED tests, including diagnostic performance, stage shift, and mortality reduction.
Several ongoing clinical trials are evaluating the effectiveness of MCED tests. These studies are key to demonstrating the clinical utility of the tests, confirming their ability to detect cancer early and improving outcomes for patients. Preliminary results from these trials indicate that MCED tests can detect several types of cancer with high specificity, although sensitivity varies. For example, trials have shown that these tests are particularly effective in detecting cancers that are currently difficult to detect using traditional screening methods, such as pancreatic and ovarian cancer.
The development and implementation of MCED tests represents significant progress in the field of cancer detection and diagnosis. These tests have the potential to revolutionize cancer screening by detecting multiple types of cancer simultaneously at an early stage. However, further research and standardization are needed to ensure their effectiveness and safety before they become a standard part of clinical practice. Continued innovation and investment in this area is vital to improve cancer survival rates and reduce the global burden of this disease.

